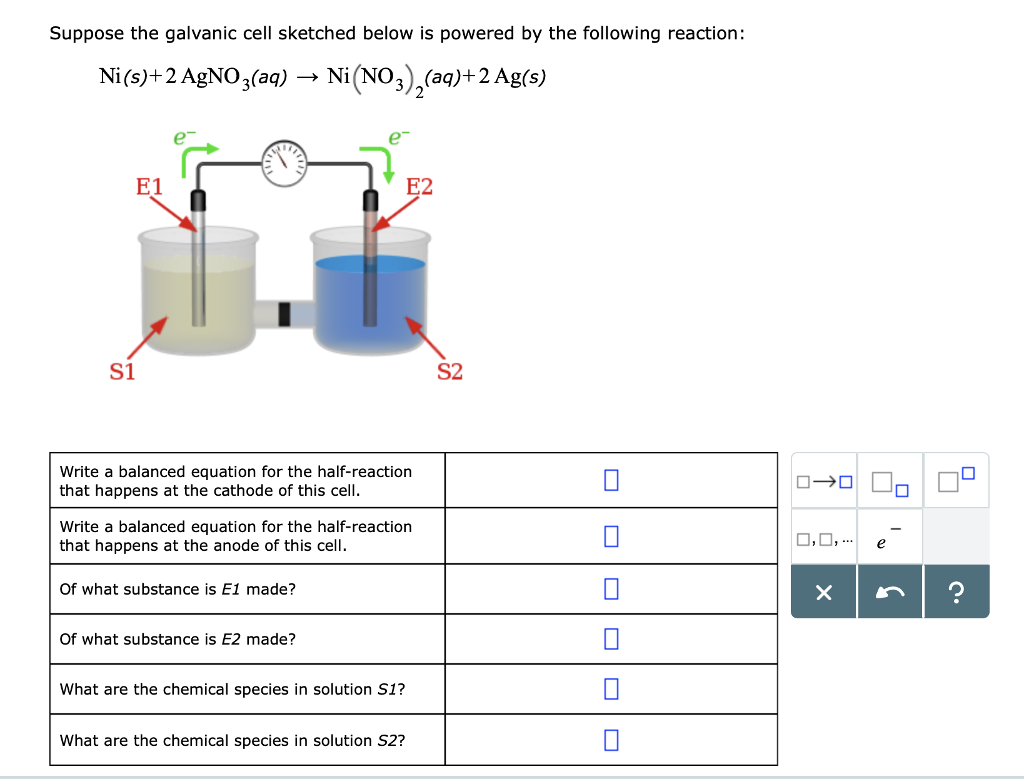
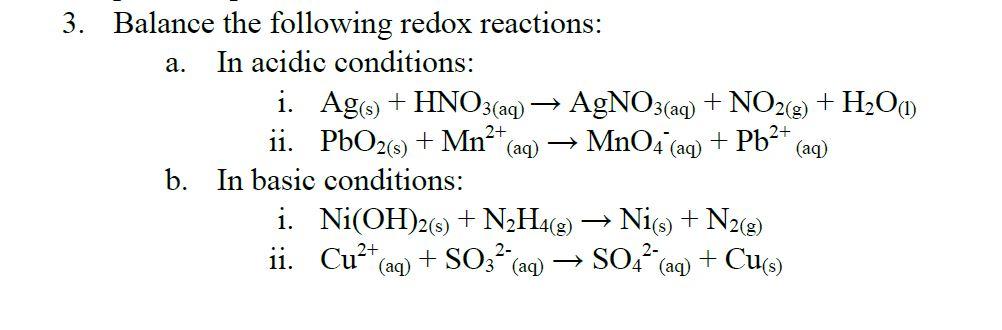3.15 A solution of Ni(NO3)2 is electrolyzed between platinum electrodes using a current of 5 amperes 20 minutes. What mass of Ni is deposited the cathode? Sol. Quantity of electricity passed =(5A) (
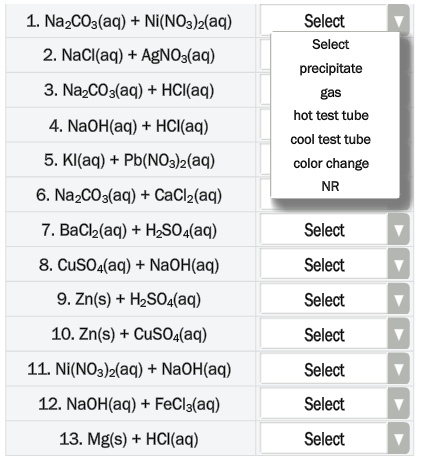
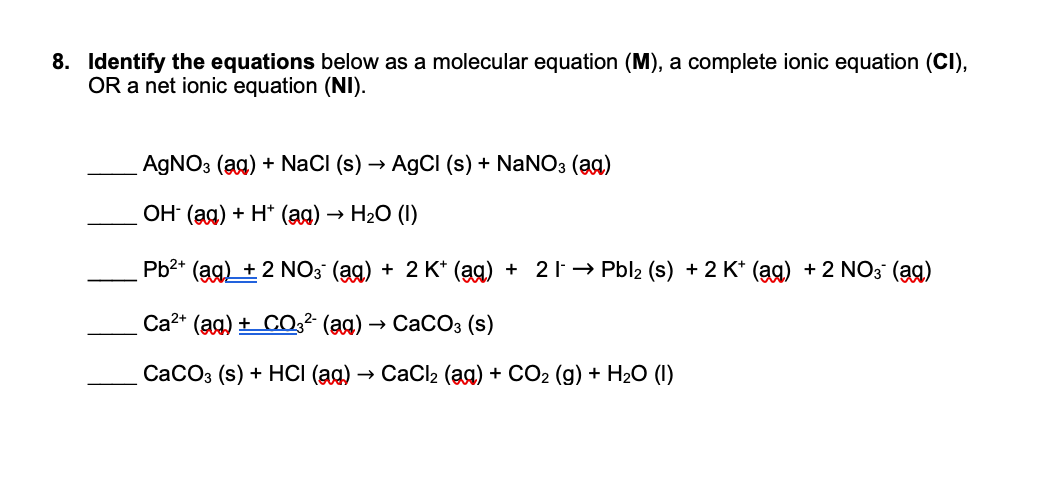
SOLVED: 1. Na2CO3(aq) + Ni(NO3)2(aq) 2. NaCl(aq) + AgNO3(aq) 3. Na2CO3(aq) + HCl(aq) â†' NaOH(aq) + CO2(g) + H2O(l) 4. KI(aq) + Pb(NO3)2(aq) â†' PbI2(s) + KNO3(aq) 5. Na2CO3(aq) + CaCl2(aq) â†'

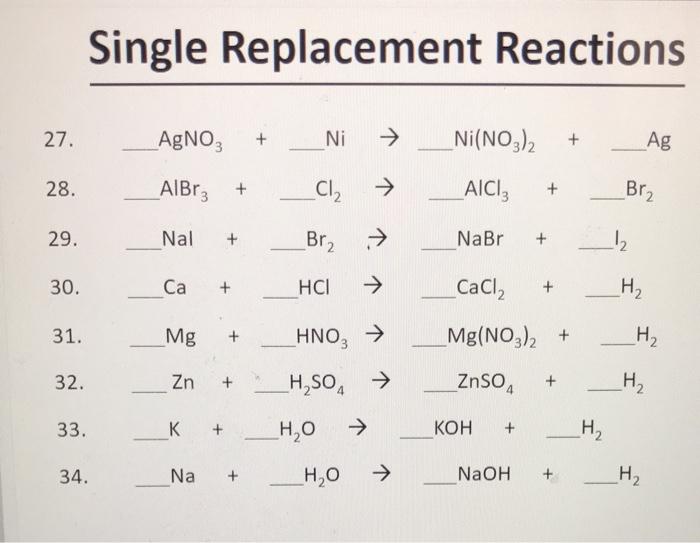
SOLVED: Select the correct balanced net ionic equation for each of the following reactions occurring in aqueous solution: Cu(NO3)2 + 2NaCl -> 2AgCl + Cu(NO3)2 Ni(NO3)2 + 2AgNO3 -> 2Ag + Ni(NO3)2
69 Hydrocarbon (A), C6H10 on treatment with H2/Ni, H2/Lindlar catalyst and Na/liquid NH3 forms three different reduction products (B), (C) and (D) respectively. (A) does not form any salt with ammoniacal AgNO3

Overcoming Limitations in Decarboxylative Arylation via Ag–Ni Electrocatalysis | Journal of the American Chemical Society
![Of the complex [Ni(NH3)Br]Cl ,the ionization isomer will give colour with AgNo3? A-White B-Red C-Yellow D-Blue.ans is option -(C) frnd .can u explain this? - EduRev NEET Question Of the complex [Ni(NH3)Br]Cl ,the ionization isomer will give colour with AgNo3? A-White B-Red C-Yellow D-Blue.ans is option -(C) frnd .can u explain this? - EduRev NEET Question](https://edurev.gumlet.io/ApplicationImages/Temp/5859434_f9efc48b-6cf4-4285-b0bc-a22082f1f486_lg.png?w=360&dpr=2.6)

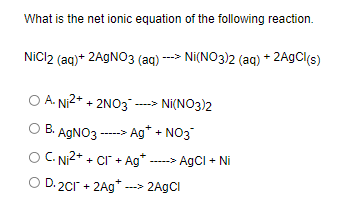

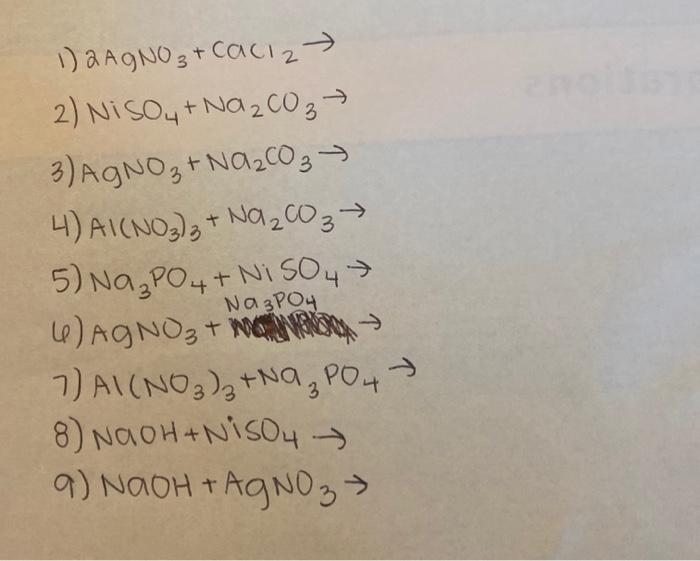





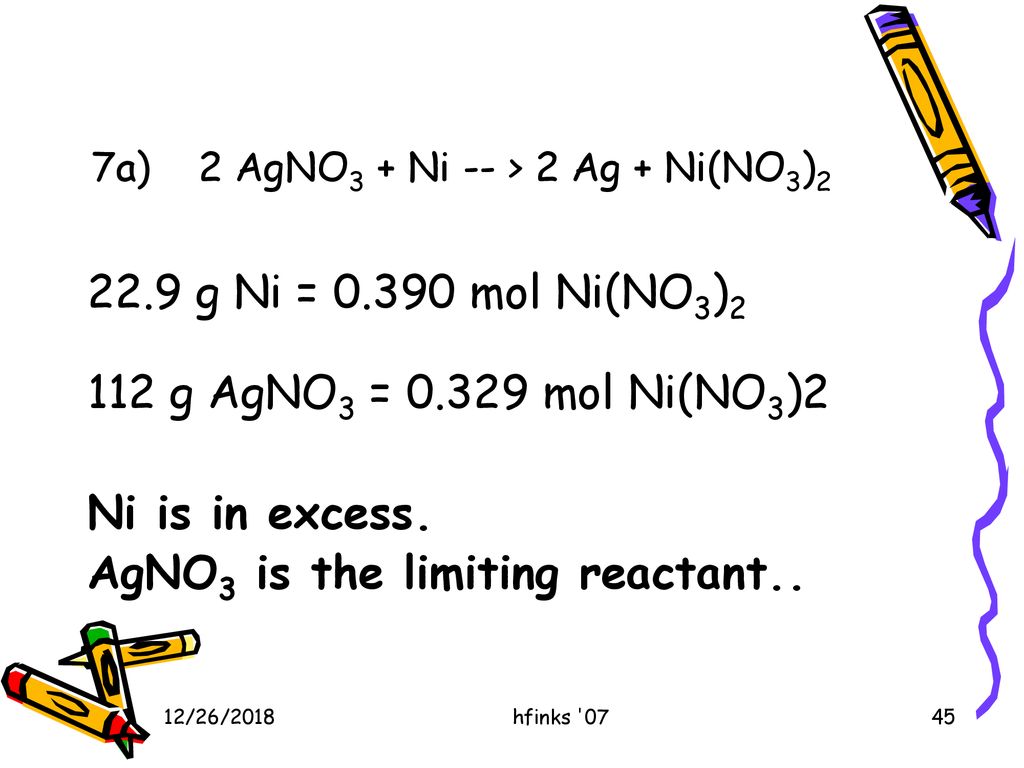
![Kannada] A strip of nickel metal is placed in a 1molar solution of Ni Kannada] A strip of nickel metal is placed in a 1molar solution of Ni](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/7678142.webp)